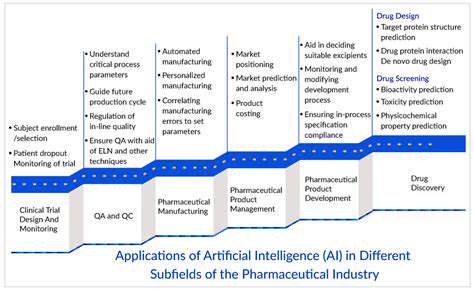
Introduction to AI-Powered Drug Manufacturing
Understanding the Potential of AI in Drug Discovery
Artificial intelligence (AI) is rapidly transforming various sectors, and the pharmaceutical industry is no exception. AI-powered drug discovery holds the promise of accelerating the development of new medications and therapies, potentially saving countless lives and improving human health. This technology allows researchers to analyze vast datasets of biological information, identify potential drug candidates, and optimize existing treatments, ultimately leading to a faster and more efficient drug development process. AI algorithms can sift through mountains of data, identifying patterns and correlations that might be missed by human researchers.
By automating repetitive tasks and increasing the speed of analysis, AI can significantly reduce the time and resources needed to bring new drugs to market. This, in turn, can lower the overall cost of drug development, making life-saving treatments more accessible to a wider population. This potential revolution in drug discovery is very exciting for the future of healthcare.
AI's Role in Identifying Potential Drug Candidates
One of the most crucial applications of AI in drug discovery is its ability to identify potential drug candidates. AI algorithms can analyze vast datasets of molecular structures, biological pathways, and clinical trial results to predict which molecules have the highest likelihood of becoming effective drugs. This process is significantly faster and more efficient than traditional methods, which often rely on laborious trial-and-error experimentation. AI can also identify previously overlooked potential drug targets, leading to breakthroughs in treating currently incurable diseases.
Machine learning algorithms, a subset of AI, are particularly adept at analyzing complex data patterns. This allows for the identification of compounds with specific binding affinities to target proteins, thereby increasing the likelihood of success in preclinical and clinical trials. This efficiency in identifying potential candidates is a major advantage of AI in this field.
Optimizing Existing Drug Therapies
AI isn't just about discovering new drugs; it's also about improving existing ones. AI algorithms can analyze existing drug data to identify ways to optimize dosage, administration methods, and even predict potential side effects. This information can lead to safer and more effective treatments. By identifying potential drug interactions, AI can also help minimize adverse effects and improve patient outcomes. Furthermore, AI can assist in tailoring treatments to individual patients based on their unique genetic makeup and other factors, maximizing efficacy and minimizing harm.
Challenges and Future Directions
While the potential of AI in drug discovery is immense, there are also challenges to overcome. Data quality and accessibility are crucial factors in the reliability of AI algorithms. Furthermore, ensuring the ethical and responsible use of AI in healthcare is paramount. Maintaining patient privacy and ensuring fair access to AI-powered treatments are critical concerns that must be addressed. Future research will likely focus on developing more sophisticated AI algorithms, improving data quality, and integrating AI tools into existing drug development workflows.
Automated Quality Control and Inspection
Automated Visual Inspection
Automated visual inspection systems are revolutionizing drug manufacturing by identifying defects and inconsistencies in tablets, capsules, and other dosage forms with remarkable speed and accuracy. These systems utilize advanced image processing algorithms to analyze images captured during various stages of the manufacturing process. This allows for the swift detection of anomalies like variations in size, shape, color, or surface texture, which might otherwise slip past human inspectors. This proactive identification of imperfections is critical to maintaining product quality and consistency, thereby reducing the risk of faulty batches reaching the market.
By automating this process, manufacturers can significantly reduce the time and resources spent on manual inspection, leading to substantial cost savings. Additionally, the data collected by these systems can be used to identify patterns and trends in defects, enabling proactive adjustments to production processes and preventing future issues. The consistent and objective nature of automated visual inspection ensures a higher level of quality control than traditional methods, ultimately leading to a safer and more effective drug supply chain.
Automated Physical Property Testing
Automated systems are now capable of rapidly and precisely measuring crucial physical properties of drug substances, such as hardness, friability, disintegration time, and dissolution rate. These tests are essential for ensuring the drug's efficacy and safety. The advanced sensors and instrumentation used in these automated systems offer superior precision and consistency compared to manual methods. These automated tests allow for real-time monitoring and feedback, enabling manufacturers to identify and address issues quickly, which can significantly improve overall production efficiency.
The ability to continuously monitor and record data on these crucial physical attributes allows for detailed analysis of the production process and identification of trends in product quality. This detailed data can be used to identify potential bottlenecks, optimize manufacturing parameters, and ultimately enhance the reliability of the drug manufacturing process. Real-time data analysis is critical for maintaining consistent product quality across large-scale production runs.
Data Analysis and Process Optimization
The wealth of data generated by automated quality control systems is not just for immediate defect identification, but also for comprehensive process optimization. AI algorithms can analyze this data to pinpoint areas where processes can be improved, leading to increased efficiency and reduced waste. By identifying correlations between various production parameters and product quality, manufacturers can make data-driven decisions to refine their procedures, minimizing deviations and maximizing yields. This iterative process of analysis and optimization leads to continuous improvement in the drug manufacturing process, resulting in higher quality products and reduced costs.
Furthermore, the data collected by these systems can be used to predict potential issues, allowing manufacturers to intervene proactively and prevent costly disruptions. The ability to analyze vast datasets allows for a deeper understanding of the manufacturing process, enabling the identification of root causes of defects and the implementation of preventative measures. This proactive approach to quality control significantly enhances the reliability and sustainability of the drug production process, ensuring a consistent supply of high-quality medications.