Predicting Drug Response with Genetic Insights
Understanding the Genetic Basis of Drug Response
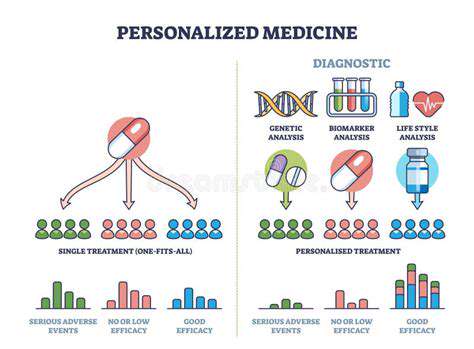
Predicting how a patient will respond to a specific drug is a crucial aspect of personalized medicine. Genetic variations play a significant role in this process, influencing how the body metabolizes and interacts with medications. Understanding these genetic variations, through methods like genome sequencing, can provide invaluable insights into potential drug efficacy and adverse reactions, paving the way for more targeted and effective treatments.
Identifying specific genetic markers associated with drug response allows clinicians to tailor treatment plans based on an individual's unique genetic makeup. This approach can lead to improved treatment outcomes, reduced side effects, and ultimately, better patient care.
The Role of Pharmacogenomics in Personalized Medicine
Pharmacogenomics is a branch of genomics that focuses on the relationship between an individual's genetic makeup and their response to drugs. This field is revolutionizing drug development and clinical practice by enabling the identification of genetic variations that influence drug metabolism, efficacy, and toxicity. By incorporating pharmacogenomic data into clinical decision-making, healthcare providers can select the most appropriate medication and dosage for each patient, maximizing therapeutic benefits and minimizing potential risks.
AI's Potential in Analyzing Complex Genetic Data
The sheer volume and complexity of genetic data necessitate sophisticated analytical tools. Artificial intelligence (AI) algorithms can process this data with unprecedented speed and accuracy, identifying patterns and correlations that might be missed by traditional methods. AI can also predict drug response based on a patient's genetic profile, potentially leading to more accurate and personalized treatment strategies.
Developing AI Models for Drug Response Prediction
Machine learning algorithms, a subset of AI, are particularly well-suited for predicting drug response. These algorithms can be trained on large datasets of genetic information and clinical outcomes to develop predictive models that identify individuals likely to benefit from specific medications.
This approach can accelerate the development of more effective and safer drugs, while also improving the efficiency of clinical trials by enabling targeted patient recruitment.
Challenges and Ethical Considerations in AI-Driven Pharmacogenomics
Despite the potential benefits, implementing AI-driven pharmacogenomics presents certain challenges. Data privacy and security are paramount considerations, as genetic information is highly sensitive. Ensuring the accuracy and reliability of AI models, particularly in diverse populations, is also crucial to avoid biases and ensure equitable access to personalized medicine.
Ethical considerations surrounding the use of genetic data in healthcare, including informed consent and patient autonomy, need careful consideration and robust regulatory frameworks.
Improving Drug Development Through Genetic Insights
Integrating genetic insights into drug development is transforming the process. By incorporating pharmacogenomic data, researchers can identify potential drug candidates with improved efficacy and reduced toxicity, minimizing the need for extensive and costly clinical trials. This approach can also accelerate the development of personalized therapies, addressing a wide range of diseases and conditions.
Future Directions and Implications for Personalized Care
The future of personalized medicine lies in the convergence of AI and pharmacogenomics. Further research and development in these areas will likely lead to even more sophisticated predictive models, enabling highly personalized treatment strategies. This approach has the potential to revolutionize healthcare by improving treatment outcomes, reducing adverse effects, and ultimately enhancing the quality of life for patients with a wide range of conditions.
As technology advances, the integration of AI and pharmacogenomics will become even more seamless, driving personalized care to new heights and ushering in a new era of precision medicine.
Accelerating Drug Design and Development
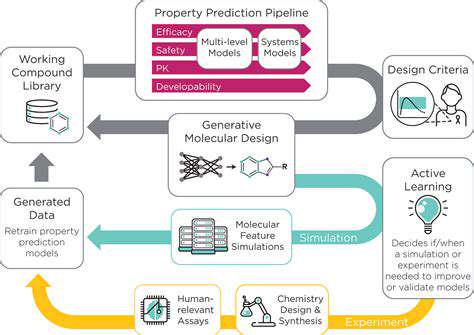
Accelerating Drug Discovery Through Computational Modeling
Computational modeling is revolutionizing the drug discovery process by enabling scientists to explore vast chemical spaces and identify promising drug candidates with unprecedented speed and efficiency. By utilizing sophisticated algorithms and powerful computers, researchers can simulate molecular interactions and predict the efficacy and safety of potential drugs long before they enter the lab. This significantly reduces the time and resources required for traditional methods, accelerating the entire drug development pipeline.
High-Throughput Screening and Automation
High-throughput screening (HTS) technologies are automating the process of testing large libraries of compounds against biological targets. This allows researchers to rapidly evaluate the potential of numerous compounds, significantly increasing the chances of identifying effective drug candidates.
Automated systems are crucial in this process, as they minimize human error and increase the consistency and reproducibility of the results. This increased efficiency is a key driver in accelerating the drug development process.
Advanced Machine Learning Algorithms
Machine learning algorithms are proving invaluable in analyzing complex biological data and identifying patterns that might otherwise be missed. These algorithms can sift through vast datasets of molecular structures, biological activity data, and clinical trial results to predict the efficacy and safety of potential drugs with remarkable accuracy. This ability to predict potential outcomes is drastically cutting down the time needed for testing and refinement.
Improved Target Identification and Validation
Identifying and validating drug targets is crucial for effective drug design. Modern techniques, including genomics and proteomics, are providing valuable insights into the biological pathways involved in disease. This deeper understanding of targets enables researchers to design more specific and effective drugs. A better understanding of the disease mechanism leads to more precise and efficient drug development efforts.
3D Structure Modeling and Drug Docking
Understanding the 3D structure of proteins and other biological targets is essential for designing drugs that can bind to them effectively. Advanced computational methods allow researchers to predict the 3D structure of proteins and simulate how different compounds interact with them. This process of drug docking helps optimize the fit between the drug and the target, increasing the likelihood of success.
Personalized Medicine and Drug Tailoring
The concept of personalized medicine is becoming increasingly important in drug development. Computational tools are now being utilized to tailor drugs to individual patients based on their genetic makeup and other characteristics. This approach can lead to more effective treatments and fewer side effects. The data generated can be used to refine treatment protocols based on individual patient responses.
Data Management and Integration
The sheer volume of data generated in drug discovery requires sophisticated data management and integration systems. These systems ensure that data from various sources, including experiments, simulations, and clinical trials, are accessible and usable by researchers. This organized access to data is essential for accelerating the analysis and ultimately speeding up the drug development process. Robust data management systems are critical for efficient and effective research.
Improving Clinical Trial Design and Patient Selection
Improving Patient Selection through AI
AI algorithms can analyze vast datasets of patient information, including medical history, genetic profiles, lifestyle factors, and imaging data, to identify individuals who are most likely to benefit from a specific treatment. This personalized approach to patient selection can significantly increase the efficiency and effectiveness of clinical trials by enrolling patients with a higher likelihood of positive treatment outcomes. By identifying patients with specific genetic markers or disease characteristics, AI can significantly improve the chances of success for the clinical trial and ultimately for the patients involved. This approach can also help reduce the time and resources required for the trials as well.
Furthermore, AI can identify potential biases in existing patient selection criteria, enabling researchers to develop more equitable and inclusive trial designs. This focus on inclusivity and fairness is crucial for ensuring that the benefits of new treatments are accessible to a diverse population. By identifying and correcting any biases in the data, AI algorithms can help create a more representative sample of participants, potentially leading to more reliable and generalizable results.
Optimizing Trial Design with Predictive Modeling
AI-powered predictive modeling can analyze trial data to identify factors influencing treatment response, allowing researchers to tailor the trial design for better outcomes. By identifying key variables associated with treatment success or failure, researchers can optimize the trial design to focus resources on more promising approaches and potentially shorten the overall trial duration. This approach is crucial for the efficient use of resources in clinical trials and can result in more impactful findings.
Predictive models can also help researchers anticipate potential challenges during the trial, such as patient attrition or adverse events. This proactive approach enables them to implement preventative measures or adjust the trial protocol to mitigate these risks, ultimately improving the overall success rate of the clinical trial and ensuring a safe environment for participants.
Enhancing Data Collection and Management
AI can automate the collection and management of clinical trial data, reducing human error and improving data quality. This automation allows for more efficient data entry, validation, and analysis, freeing up researchers to focus on more complex tasks, such as interpreting results and developing new hypotheses. Improved data management through AI can lead to reduced data errors and faster analysis, enabling researchers to reach conclusions more quickly.
By automating various aspects of data management, AI can also minimize the risk of data loss or corruption. This increased data security and reliability is vital in clinical trials, where the integrity of the data is paramount to the validity of the results. Ultimately, this leads to more reliable and trustworthy clinical trial findings.
Utilizing AI for Real-World Evidence
AI can analyze real-world data from electronic health records, insurance claims, and other sources to gain insights into treatment effectiveness and safety in diverse populations. This approach can identify patterns and trends that may not be apparent in traditional clinical trials, providing valuable insights to researchers and clinicians. By incorporating real-world data, researchers can potentially identify more comprehensive information about the effects and safety of the treatment in various populations and contexts, leading to more informed and reliable conclusions.
This analysis of real-world data can also help identify potential safety concerns or treatment limitations that may not be evident in a controlled clinical trial environment. This real-world perspective is crucial for refining treatment strategies and ensuring that the developed treatments are safe and effective for diverse patient populations.
Enhancing Drug Safety and Efficacy Through Simulation

Improving Clinical Trial Design
Rigorous clinical trial design is paramount for evaluating drug safety and efficacy. Careful consideration of the patient population, appropriate control groups, and comprehensive outcome measures are crucial for robust data collection. This meticulous approach minimizes bias and ensures reliable results, leading to more accurate assessments of a drug's potential benefits and risks. Using advanced statistical methods and incorporating diverse patient populations into trials is vital for generalizability and wider applicability of the findings.
Optimizing Pharmacokinetic and Pharmacodynamic Studies
Understanding how a drug interacts with the body, its absorption, distribution, metabolism, and excretion (ADME), is critical. Detailed pharmacokinetic studies provide insights into the drug's behavior within the body, influencing dosing strategies and potentially identifying adverse reactions. Pharmacodynamic studies, evaluating the drug's effects on biological systems, are equally important for predicting therapeutic efficacy and potential side effects. Combining both approaches allows for a more comprehensive understanding of a drug's mechanism of action and its impact on the body.
Employing Advanced Analytical Techniques
Sophisticated analytical methods are essential for precise drug characterization. High-performance liquid chromatography (HPLC) and mass spectrometry (MS) are invaluable tools for identifying and quantifying drug compounds and metabolites, ensuring drug quality and consistency. This precision is vital for safety, as impurities or variations in drug formulations can impact efficacy and introduce potential risks. These techniques also aid in understanding drug interactions and optimizing therapeutic strategies.
Advancing Safety Monitoring Strategies
Proactive and comprehensive safety monitoring throughout the drug development lifecycle is critical. Post-marketing surveillance, utilizing large databases and real-world data, is essential for detecting rare or delayed adverse events not apparent during clinical trials. This proactive approach allows for swift identification and management of potential risks. Utilizing signal detection methodology and predictive modeling can further enhance the efficiency of safety monitoring.
Integrating Patient-Oriented Research
Incorporating patient perspectives and experiences into drug development is vital. Collecting patient feedback through surveys, focus groups, and patient advisory boards can provide valuable insights into real-world needs and concerns. This patient-centric approach can facilitate the development of more effective and acceptable treatments. Understanding the impact of a drug on patients' lives beyond just clinical outcomes is key to improving overall patient care.
Promoting Transparency and Collaboration
Open communication and collaboration among researchers, regulators, and industry stakeholders are crucial for accelerating drug development and ensuring safety. Transparency in data sharing and research methodologies fosters trust and allows for peer review and validation. Sharing knowledge across disciplines enhances the overall quality of drug development processes. Collaborative approaches can address complex challenges and accelerate the translation of research into impactful treatments.
Leveraging Big Data and Artificial Intelligence
Big data analytics and artificial intelligence (AI) offer powerful tools for accelerating drug discovery and development. Analyzing large datasets can identify patterns and predict potential drug candidates, reducing the time and cost of research. AI algorithms can also predict potential drug interactions and adverse events. These technologies can be instrumental in identifying novel therapeutic targets and optimizing treatment strategies for specific patient populations.